UCR: Huinan Liu (lead), Devin Binder & Guillermo Aguilar— Thrust 4
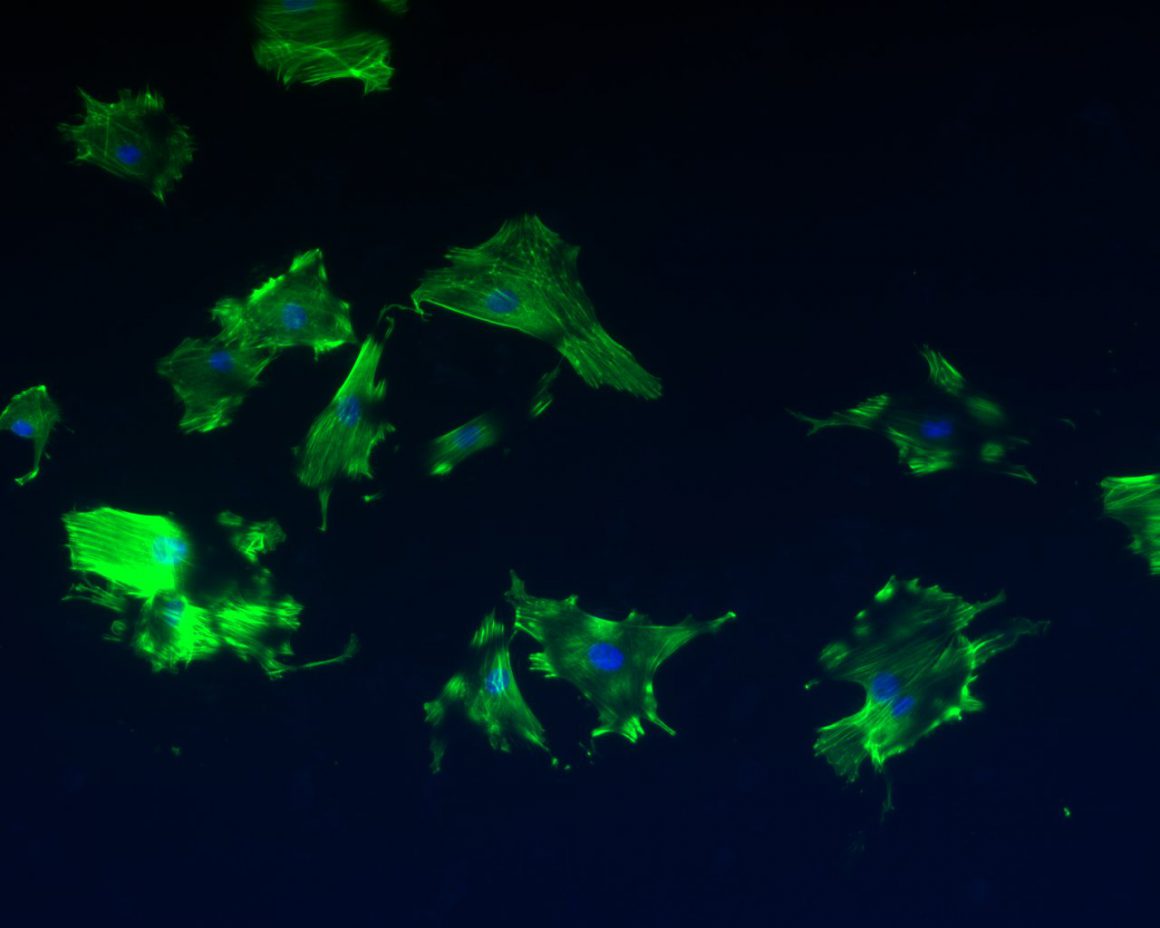
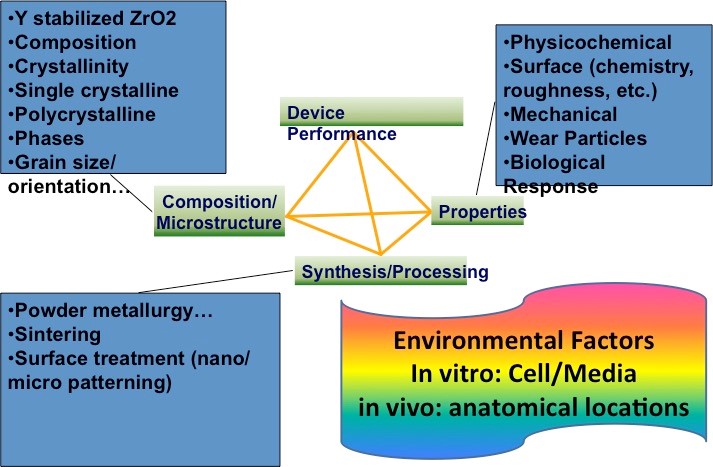
Scientific/technological challenges: Although conventionally-processed microcrystalline YSZ (mc-YSZ) has well-proven biocompatibility in dental and orthopedic applications, through assessment within the context of cranial implants is lacking and is necessitated by the environment’s unique demands (e.g. intracranial pulsatility, cerebrospinal fluid exposure, etc.). Biocompatibility assessments for nc-YSZ specifically, also lacking, are necessitated by the unique properties associated with nanocrystallinity (e.g. increased surface energy). Finally, while others have reported that nc-YSZ is resistant to the aging-induced degradation observed in more conventional mc-YSZ implants (i.e. leaching-induced destabilization of the cubic &/or tetragonal phase), independent assessments are required for our nc-YSZ materials specifically, since they possess grain sizes well below that in earlier studies, along with increased density and reduced porosity, both of which may further improve aging resistance. The envisioned WttB platform should be able to support angiogenesis and neovascularization. While angiogenesis is mainly characterized by the protrusion and outgrowth of capillary buds and sprouts from pre-existing blood vessels, neovascularization forms microvascular networks with red blood cell (RBC) perfusion. Both processes are required to ensure successful engraftment into host tissue. Biocompatibility demands that, these materials should not induce severe local or systemic inflammatory responses. Interaction of implanted biomaterials with host tissue is dynamic and comprises distinct steps. Immediately following implantation, a surface layer of proteins forms onto the surface of the implant, which critically influences the subsequent cellular reaction to the implant. Activated leukocytes infiltrate the implantation site and produce various cytokines and growth factors. Moreover, monocytes recruited from the blood stream begin to differentiate into macrophages. Inflammation can result in a chronic foreign body reaction associated with lymphocyte accumulation and fusion of individual macrophages into multinucleated giant cells, typically found at the interface between the implant and surrounding host tissue. Lastly, the implant induces fibroblast proliferation and migration, as well as neovascularization or fibrous encapsulation. Since a foreign body reaction involves a range of cell types, cytokines and growth factors, it is impossible to fully simulate this complex process in vitro, necessitating in vivo analysis.
Scientific/technological goals: The goal of this thrust is to develop both in vitro and in vivo models to evaluate the biocompatibility of the WttB platform at varying developmental stages, including: a) initial in vitro models to determine baseline nc-YSZ cytocompatibility and aging resistance, and evaluate cellular responses to nano-scale features and patterns; b) small animal studies to evaluate skull integration and inflammatory responses; and c) small animal studies to evaluate integrated device functionality. Collateral scientific studies: These models will facilitate future standardization of biocompatibility testing protocols for brain-related devices emerging from the BRAIN initiative, and for which protocols and guidelines DO NOT currently exist. Testing guidelines and protocols for BRAIN device biocompatibility at various developmental stages following implantation would pioneer standards for the biomedical community.
Envisioned fit with other topics: This thrust tightly integrates with nanopowders synthesis, implant fabrication and nanoscale net-shape forming, optical fiber coupling, and laser-written photonic structures.

Leave a Reply