INAOE: Rubén Ramos-García (lead); UNAM: Juan Hernández- Cordero; INAOE: Santiago Camacho-López; UCR: Guillermo Aguilar — Thrust 4
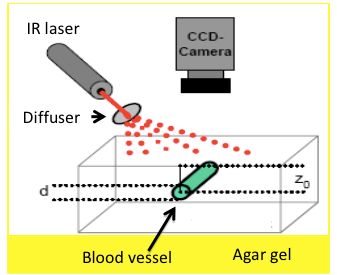
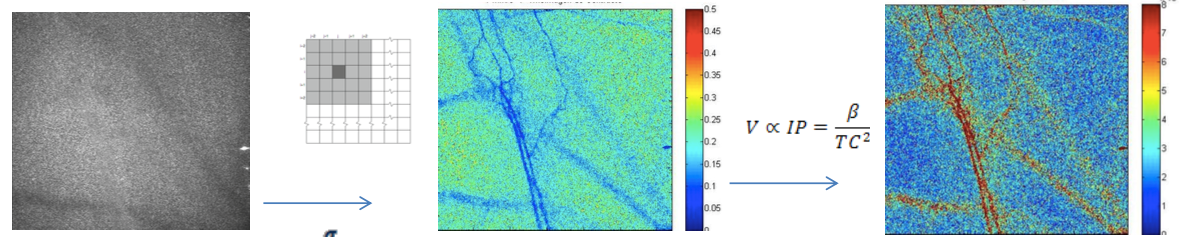
Scientific/technological challenges: Optical imaging and therapeutic techniques for neurological applications continue to be hindered by the inability to deliver the necessary light in situ, on demand, over large areas, and on a chronically-recurring basis. A cranial implant platform proposed, with optical transparency, waveguides and optical fiber couplings, would enable mechanical, thermal, optical, chemical interrogation and stimulation of tissue targets in the near-surface, mid, and deep regions of brain tissue. For example, in optogenetics, this platform would deepen spatial optical access for simultaneous visualization/therapy of subsurface and deep tissue targets with minimal invasiveness. In the area of neuromodulation, laser-induced heating has been demonstrated as effective for neural inhibition within a hybrid electrical stimulation/thermal device, for particle-based photothermal therapy, and for laser-induced thermal ablation of brain tumors. For these and many others, optical visualization offers guidance on structure, structural abnormalities and therapeutic improvements. For example, a very successful strategy in imaging through scattering materials is to separate the small amount of light that does not change direction owing to random scattering (ballistic light) from the scattered light background using a time-gated technique, such as optical coherence tomography (OCT). In fact, using OCT, we demonstrated recently the initial feasibility of nc-YSZ cranial implants by imaging neural structures in an acute in vivo murine model with greater depth and resolution than through native cranium. Unfortunately, OCT is expensive, complicated, and its resolution (typically between 15-20 μm) does not provide the detail necessary to guide many brain diagnostics and laser therapies. A technique that would benefit from accurate optical interrogation of the brain is photodynamic therapy (PDT), particularly for brain tumor treatments. However, improved efficacy of in vivo studies relies on correlation of three key factors: 1) drug (photosensitizer) dose; 2) light dose; and 3) oxygen concentration. Visualization of blood vessels – critical for vital health statistics, such as biocompatibility of implant material, oxygenation or the efficacy of medical treatment — is difficult because the scalp and skull act as strongly scattering media. Of particular importance is neuron oxygen deprivation, which may lead to irreversible damage and fatal consequences. We propose that the WttB platform, coupled with laser speckle imaging (LSI), would help determine optimal PDT parameters by providing a real-time measure of relative blood flow speed changes and correlation of oxygen concentration and light dose. LSI uses the spatial statistics of time-integrated speckle (essentially the speckle contrast), which was originally developed to measure retinal blood flow. However, more recently, LSI has been used to noninvasively monitor blood flow and perfusion dynamics in the brain and skin. In particular, LSI has been applied in skin to monitor blood flow dynamics during PDT.
Scientific/technological goals: We will develop a LSI system with high spatial and temporal resolution, capable of visualizing in an inexpensive, label-free and, non-invasive way, cortical dynamic blood flow and blood oxygenation. This system should be able to acquire wide-field speckle images and work with spatial and temporal algorithms developed to improve its sensitivity. Collateral scientific studies: Aside from the studies proposed, the WttB platform will enable long term (few weeks to months) in vivo studies of therapeutic techniques for brain pathologies, such as those required to study brain hemodynamics in cerebral malaria. Our collaborators (Park and Binder) have demonstrated that OCT provides a means for neuroimaging in vivo using a thinned skull murine model. While offering significant promise for acute settings, the current requirement for skull thinning remains an impediment to chronic monitoring. The WttB concept could address this challenge. Although beyond the scope of the proposed effort, WttB could enable direct, real-time monitoring of imaging biomarkers for chronic traumatic encephalopathy, and allow tracking of changes currently only measurable by histology post mortem. Similarly, chronic optical monitoring and/or more precisely targeted photodynamic treatment of residual gliomas could prolong survival and improve quality of life for brain cancer patients. Finally, WttB could provide an enabling platform to extend emerging optogenetic neuromodulation techniques toward chronic studies in humans; leading to clinical applications ranging from neurology to psychiatry.
Envisioned fit with other topics: Laser therapy studies, part of the translational research thrust of this proposal, are intimately integrated with the diagnostic and biocompatibility studies. In particular, many of the laser-therapy studies aim at measuring the spatiotemporal distribution of heat, blood flow velocity, and pressure in the therapy target and adjacent tissue, using infrared thermal imaging (e.g., FLIR A320), interferometric-based techniques (e.g., time-resolved Mach–Zehnder interferometry) and piezoelectric sensing (e.g., piezoelectric homemade polyvinylidene fluoride (PVDF) transducers).

Leave a Reply